If You Made A Change In The Promoter Sequence In The Dna, What Would Happen At The Rna Level?
If Deoxyribonucleic acid is a book, then how is it read? Learn more virtually the DNA transcription process, where DNA is converted to RNA, a more portable set of instructions for the cell.
The genetic code is oftentimes referred to every bit a "blueprint" considering it contains the instructions a cell requires in order to sustain itself. We at present know that there is more to these instructions than simply the sequence of letters in the nucleotide code, however. For example, vast amounts of evidence demonstrate that this lawmaking is the basis for the production of various molecules, including RNA and poly peptide. Research has also shown that the instructions stored within DNA are "read" in two steps: transcription and translation. In transcription, a portion of the double-stranded DNA template gives ascent to a unmarried-stranded RNA molecule. In some cases, the RNA molecule itself is a "finished production" that serves some important role inside the cell. Often, however, transcription of an RNA molecule is followed by a translation stride, which ultimately results in the production of a poly peptide molecule.
Visualizing Transcription
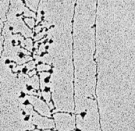
The process of transcription tin be visualized by electron microscopy (Figure 1); in fact, information technology was first observed using this method in 1970. In these early electron micrographs, the DNA molecules appear as "trunks," with many RNA "branches" extending out from them. When DNAse and RNAse (enzymes that dethrone Deoxyribonucleic acid and RNA, respectively) were added to the molecules, the application of DNAse eliminated the trunk structures, while the employ of RNAse wiped out the branches.
Deoxyribonucleic acid is double-stranded, but but one strand serves as a template for transcription at whatever given time. This template strand is called the noncoding strand. The nontemplate strand is referred to as the coding strand considering its sequence will be the same as that of the new RNA molecule. In most organisms, the strand of DNA that serves equally the template for 1 cistron may be the nontemplate strand for other genes within the same chromosome.
The Transcription Process
The process of transcription begins when an enzyme called RNA polymerase (RNA pol) attaches to the template DNA strand and begins to catalyze production of complementary RNA. Polymerases are large enzymes composed of approximately a dozen subunits, and when active on DNA, they are as well typically complexed with other factors. In many cases, these factors signal which gene is to be transcribed.
Three dissimilar types of RNA polymerase be in eukaryotic cells, whereas bacteria have simply i. In eukaryotes, RNA pol I transcribes the genes that encode most of the ribosomal RNAs (rRNAs), and RNA pol Three transcribes the genes for ane pocket-sized rRNA, plus the transfer RNAs that play a primal office in the translation process, too as other small regulatory RNA molecules. Thus, it is RNA pol II that transcribes the messenger RNAs, which serve as the templates for production of protein molecules.
Transcription Initiation

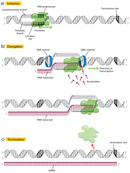
The first step in transcription is initiation, when the RNA political leader binds to the DNA upstream (5′) of the factor at a specialized sequence called a promoter (Figure 2a). In leaner, promoters are usually composed of iii sequence elements, whereas in eukaryotes, there are as many as seven elements.
In prokaryotes, almost genes have a sequence called the Pribnow box, with the consensus sequence TATAAT positioned well-nigh x base pairs away from the site that serves as the location of transcription initiation. Not all Pribnow boxes have this exact nucleotide sequence; these nucleotides are but the most mutual ones found at each site. Although substitutions do occur, each box notwithstanding resembles this consensus fairly closely. Many genes also take the consensus sequence TTGCCA at a position 35 bases upstream of the start site, and some have what is called an upstream element, which is an A-T rich region twoscore to 60 nucleotides upstream that enhances the charge per unit of transcription (Effigy 3). In any case, upon binding, the RNA pol "cadre enzyme" binds to another subunit called the sigma subunit to form a holoezyme capable of unwinding the DNA double helix in order to facilitate access to the cistron. The sigma subunit conveys promoter specificity to RNA polymerase; that is, it is responsible for telling RNA polymerase where to demark. There are a number of different sigma subunits that bind to different promoters and therefore assist in turning genes on and off as conditions alter.
Eukaryotic promoters are more complex than their prokaryotic counterparts, in role considering eukaryotes have the aforementioned three classes of RNA polymerase that transcribe different sets of genes. Many eukaryotic genes also possess enhancer sequences, which can be found at considerable distances from the genes they affect. Enhancer sequences command gene activation past binding with activator proteins and altering the 3-D construction of the Dna to help "concenter" RNA pol II, thus regulating transcription. Because eukaryotic DNA is tightly packaged every bit chromatin, transcription also requires a number of specialized proteins that help brand the template strand accessible.
In eukaryotes, the "core" promoter for a gene transcribed by pol 2 is well-nigh oft found immediately upstream (5′) of the start site of the gene. Most political leader Ii genes have a TATA box (consensus sequence TATTAA) 25 to 35 bases upstream of the initiation site, which affects the transcription rate and determines location of the start site. Eukaryotic RNA polymerases use a number of essential cofactors (collectively called general transcription factors), and one of these, TFIID, recognizes the TATA box and ensures that the correct start site is used. Another cofactor, TFIIB, recognizes a dissimilar common consensus sequence, G/C 1000/C G/C G C C C, approximately 38 to 32 bases upstream (Figure 4).
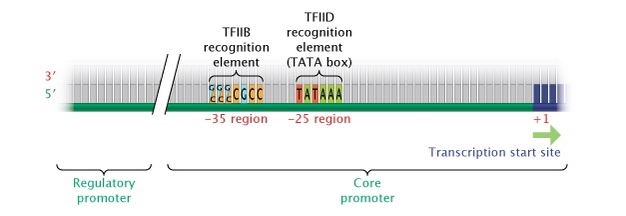
Figure iv: Eukaryotic core promoter region.
In eukaryotes, genes transcribed into RNA transcripts by the enzyme RNA polymerase 2 are controlled by a core promoter. A cadre promoter consists of a transcription beginning site, a TATA box (at the -25 region), and a TFIIB recognition chemical element (at the -35 region).
© 2014 Nature Education Adjusted from Pierce, Benjamin. Genetics: A Conceptual Approach, 2nd ed. All rights reserved. ![]()
The terms "strong" and "weak" are ofttimes used to describe promoters and enhancers, according to their effects on transcription rates and thereby on factor expression. Alteration of promoter strength can have deleterious effects upon a prison cell, often resulting in disease. For example, some tumor-promoting viruses transform healthy cells by inserting strong promoters in the vicinity of growth-stimulating genes, while translocations in some cancer cells place genes that should be "turned off" in the proximity of potent promoters or enhancers.
Enhancer sequences practise what their name suggests: They act to raise the rate at which genes are transcribed, and their effects tin can exist quite powerful. Enhancers can exist thousands of nucleotides away from the promoters with which they interact, but they are brought into proximity by the looping of DNA. This looping is the event of interactions between the proteins bound to the enhancer and those bound to the promoter. The proteins that facilitate this looping are called activators, while those that inhibit it are chosen repressors.
Transcription of eukaryotic genes by polymerases I and Three is initiated in a similar manner, but the promoter sequences and transcriptional activator proteins vary.
Strand Elongation
Once transcription is initiated, the Dna double helix unwinds and RNA polymerase reads the template strand, adding nucleotides to the 3′ end of the growing chain (Effigy 2b). At a temperature of 37 degrees Celsius, new nucleotides are added at an estimated charge per unit of about 42-54 nucleotides per second in leaner (Dennis & Bremer, 1974), while eukaryotes proceed at a much slower stride of approximately 22-25 nucleotides per second (Izban & Luse, 1992).
Transcription Termination
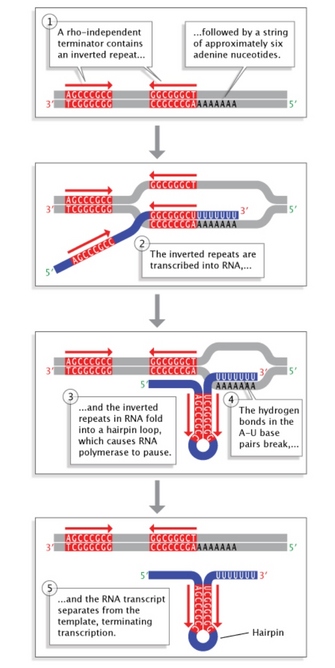
Effigy five: Rho-contained termination in bacteria.
Inverted echo sequences at the end of a factor allow folding of the newly transcribed RNA sequence into a hairpin loop. This terminates transcription and stimulates release of the mRNA strand from the transcription machinery.
© 2014 Nature Instruction Adapted from Pierce, Benjamin. Genetics: A Conceptual Approach, 2d ed. All rights reserved. ![]()
Terminator sequences are found close to the ends of noncoding sequences (Figure 2c). Bacteria possess two types of these sequences. In rho-independent terminators, inverted echo sequences are transcribed; they tin then fold back on themselves in hairpin loops, causing RNA pol to pause and resulting in release of the transcript (Figure 5). On the other hand, rho-dependent terminators make apply of a factor called rho, which actively unwinds the DNA-RNA hybrid formed during transcription, thereby releasing the newly synthesized RNA.
In eukaryotes, termination of transcription occurs past unlike processes, depending upon the exact polymerase utilized. For politico I genes, transcription is stopped using a termination factor, through a mechanism similar to rho-dependent termination in leaner. Transcription of pol Three genes ends after transcribing a termination sequence that includes a polyuracil stretch, by a machinery resembling rho-independent prokaryotic termination. Termination of pol II transcripts, yet, is more circuitous.
Transcription of pol 2 genes tin can continue for hundreds or even thousands of nucleotides across the end of a noncoding sequence. The RNA strand is then broken by a circuitous that appears to associate with the polymerase. Cleavage seems to exist coupled with termination of transcription and occurs at a consensus sequence. Mature pol Two mRNAs are polyadenylated at the iii′-end, resulting in a poly(A) tail; this process follows cleavage and is also coordinated with termination.
Both polyadenylation and termination make use of the same consensus sequence, and the interdependence of the processes was demonstrated in the late 1980s by work from several groups. Ane group of scientists working with mouse globin genes showed that introducing mutations into the consensus sequence AATAAA, known to exist necessary for poly(A) addition, inhibited both polyadenylation and transcription termination. They measured the extent of termination by hybridizing transcripts with the dissimilar poly(A) consensus sequence mutants with wild-type transcripts, and they were able to see a decrease in the signal of hybridization, suggesting that proper termination was inhibited. They therefore concluded that polyadenylation was necessary for termination (Logan et. al., 1987). Another group obtained similar results using a monkey viral system, SV40 (simian virus 40). They introduced mutations into a poly(A) site, which caused mRNAs to accumulate to levels far to a higher place wild type (Connelly & Manley, 1988).
The verbal relationship betwixt cleavage and termination remains to be adamant. 1 model supposes that cleavage itself triggers termination; another proposes that polymerase activity is affected when passing through the consensus sequence at the cleavage site, perhaps through changes in associated transcriptional activation factors. Thus, research in the area of prokaryotic and eukaryotic transcription is nevertheless focused on unraveling the molecular details of this complex process, data that volition allow us to improve empathise how genes are transcribed and silenced.
References and Recommended Reading
Connelly, S., & Manley, J. 50. A functional mRNA polyadenylation signal is required for transcription termination past RNA polymerase II. Genes and Development iv, 440–452 (1988)
Dennis, P. P., & Bremer, H. Differential rate of ribosomal protein synthesis in Escherichia coli B/r. Journal of Molecular Biology 84, 407–422 (1974)
Dragon. F., et al. A big nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417, 967–970 (2002) doi:10.1038/nature00769 (link to article)
Izban, Yard. Yard., & Luse, D. S. Factor-stimulated RNA polymerase II transcribes at physiological elongation rates on naked Dna simply very poorly on chromatin templates. Periodical of Biological Chemical science 267, 13647–13655 (1992)
Kritikou, Eastward. Transcription elongation and termination: It ain't over until the polymerase falls off. Nature Milestones in Gene Expression 8 (2005)
Lee, J. Y., Park, J. Y., & Tian, B. Identification of mRNA polyadenylation sites in genomes using cDNA sequences, expressed sequence tags, and trace. Methods in Molecular Biology 419, 23–37 (2008)
Logan, J., et al. A poly(A) improver site and a downstream termination region are required for efficient abeyance of transcription by RNA polymerase Two in the mouse beta maj-globin gene. Proceedings of the National University of Sciences 23, 8306–8310 (1987)
Nabavi, S., & Nazar, R. N. Nonpolyadenylated RNA polymerase II termination is induced by transcript cleavage. Journal of Biological Chemistry 283, 13601–13610 (2008)
Source: https://www.nature.com/scitable/topicpage/dna-transcription-426/
Posted by: woodsbobviscep.blogspot.com


0 Response to "If You Made A Change In The Promoter Sequence In The Dna, What Would Happen At The Rna Level?"
Post a Comment